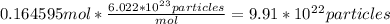Answer: 9.91×10²³ particles
Step-by-step explanation:
To find the amount of particles, you will need to use the Ideal Gas Law with what we are given.
Ideal Gas Law: PV=nRT
After we find moles, we can use Avogadro's number to convert to particles.
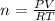
P=101.3kPa=1.00 atm
V=4.0 L
T=23°C+273.15=296.15 K
R=0.08206 Latm/Kmol
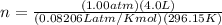
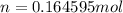
Now that we have moles, we can convert to particles.