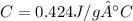Answer: 0.424 J/g°C
Step-by-step explanation:
For this problem, we would have to manipulate the equaiton for heat, q=mCT. Specific heat is the C in the equation. Since we are looking for specific heat, we manipulate the equation so that it says C=.
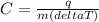
*I didn't know how to type in delta so I just wrote the word delta, but pretend you see a Δ.
Now that we have our equation, we can plug in our values and solve.
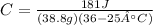
*Please ignore the capital A in the equation. It pops up every time I type in the ° sign.