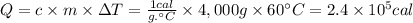Answer:
2.4 × 10⁵ cal
Step-by-step explanation:
Step 1: Given data
- Initial temperature: 20°C
- Specific heat capacity of water (c): 1 cal/g.°C
Step 2: Convert the mass to grams
We will use the relationship 1 kg = 1,000 g.
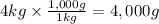
Step 3: Calculate the change in the temperature
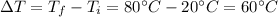
Step 4: Calculate the heat required (Q)
We will use the following expression.