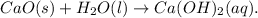Answer:
Following are the answer to this question:
Step-by-step explanation:
(1) Calcium oxide (CaO) is also referred to as quicklime. It is the solution for the substance used for whitewashing.
Whenever it reacts with water (
 ) and absorbs carbon dioxide (
) and absorbs carbon dioxide (
 ), it then formed calcium carbonate (
), it then formed calcium carbonate (
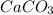 ), which produced a hard laminate on the walls, to first be whitened by the production of calcium hydroxide (CaOH).
), which produced a hard laminate on the walls, to first be whitened by the production of calcium hydroxide (CaOH).
(2) Slackened lime or calcium hydroxide arises whenever the fast limestone reacts with water.