Answer:
Step-by-step explanation:
From the given information:
Carbon carbon double bonds , for example butene are attacked by electrophiles (electron deficient species that are capable of withdrawing electrons from electron rich centers) e.g HBr
So if HBr reacts with butene and an electrophilic addition reaction takes place , a product known as 2 - bromobutane is being formed.
BUT, in the case of diethylamine; popularly known as Et₂NH(a nucleophile) no reaction is formed.
i.e Et₂NH + butene → NO REACTION
However; when the carbon-carbon double bond has a carbonyl group adjacent to it, it will turn to a carbonyl compound known as alkanone(ketones).
Now when Et₂NH react with buten-2-one , a reaction is formed. This type of reaction is Known as MIchael Reaction.
where the base in the reaction attracts a proton from the amine compound to form and ion known as amine ion which is a strong nucleophile
However, the nucleophile then in turns attack the
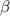 - carbon of the conjugate thereby resulting into a resonance stabilized enolate anion.
- carbon of the conjugate thereby resulting into a resonance stabilized enolate anion.
The structure of this resonance contributor explained above is shown in the image attached below.