Answer:
A
Step-by-step explanation:
Recall that ΔH is the sum of the heats of formation of the products minus the heat of formation of the reactants multiplied by their respective coefficients. That is:
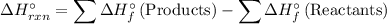
Therefore, from the chemical equation, we have that:
![\displaystyle \begin{aligned} (-317\text{ kJ/mol}) = \left[\Delta H^\circ_f \text{ N$_2$H$_4$} + \Delta H^\circ_f \text{ H$_2$O} \right] -\left[3 \Delta H^\circ_f \text{ H$_2$}+\Delta H^\circ_f \text{ N$_2$O}\right] \end{aligned}](https://img.qammunity.org/2023/formulas/chemistry/college/9a966bjqxcyfymbkwbsp09od517e9ht9et.png)
Remember that the heat of formation of pure elements (e.g. H₂) are zero. Substitute in known values and solve for hydrazine:
![\displaystyle \begin{aligned} (-317\text{ kJ/mol}) & = \left[ \Delta H^\circ _f \text{ N$_2$H$_4$} + (-285.8\text{ kJ/mol})\right] -\left[ 3(0) + (82.1\text{ kJ/mol})\right] \\ \\ \Delta H^\circ _f \text{ N$_2$H$_4$} & = (-317 + 285.8 + 82.1)\text{ kJ/mol} \\ \\ & = 50.9\text{ kJ/mol} \end{aligned}](https://img.qammunity.org/2023/formulas/chemistry/college/gspqje5bc4rmi2e8k7cqybhu2uj4mdif3m.png)
In conclusion, our answer is A.