Answer:
101
Step-by-step explanation:
Provided that
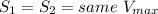
And,
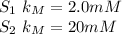
Now we expect the same
{S} (0.1mM)
This determines that
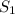 generates a higher rate of product formation as compared to the
generates a higher rate of product formation as compared to the
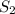
So we can easily calculate the
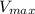 for either of
for either of
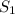 or
or
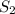 as we know that Tube 1 is
as we know that Tube 1 is
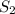 and tube 2 is
and tube 2 is
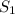
As we know that
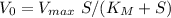
As the rates do not include any kind of units so we do not consider the units for
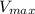
Now the calculation is
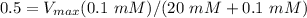
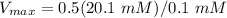
= 100.5
≈ 101