Answer:
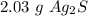
Step-by-step explanation:
For this question we have to start with the reaction:

Now, we can balance the reaction, so:

With this in mind, we have to start with the amount of
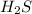 . The first step is to convert from grams to moles. For this, we need to find the molar mass of
. The first step is to convert from grams to moles. For this, we need to find the molar mass of
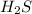 . If we check the periodic table we will find the atomic masses for Ag and H; H: 1 g/mol and A: 32 g/mol, so:
. If we check the periodic table we will find the atomic masses for Ag and H; H: 1 g/mol and A: 32 g/mol, so:
(1*2)+ (32*1) = 34 g/mol.
Now we can calculate the moles of
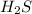 :
:
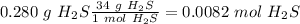
With the moles of
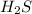 we can calculate the moles of
we can calculate the moles of
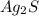 if we check the molar ratio in the balanced equation,
if we check the molar ratio in the balanced equation,
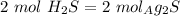 , so:
, so:
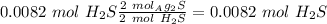
With the molar mass of
 we can convert from moles to grams (Ag: 107.86 g/mol, S: 32 g/mol), so:
we can convert from moles to grams (Ag: 107.86 g/mol, S: 32 g/mol), so:
(107.86*1)+(32*2)=247.80 g/mol
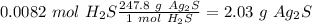
I hope it helps!