Answer:
The reaction moves to the left. To the reactants side
Step-by-step explanation:
The reaction given by the problem is:
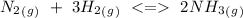
Therefore the equilibrium constant expression would be:
![K_e_q=([NH_3]^2)/([N_2][H_2]^3)](https://img.qammunity.org/2021/formulas/chemistry/college/dwvqux45iqq8so56oka6ny286a7wory0nx.png)
With the values equilibrium concentration values we can calculate the quotient "Qc", so:
![Q_c=([5.27X10^-^4]^2)/([1.97X10^-^2][3.82X10^-^2]^3)=0.251](https://img.qammunity.org/2021/formulas/chemistry/college/l18jydwqm7mxfkfw5oasxncectphtp29s0.png)
In this case Qc>Kc we will have more amount of product. Therefore, the reaction will go to the left to reach the equilibrium.
I hope it helps!