Answer:
a. 4.21 moles
b. 478.6 m/s
c. 1.5 times the root mean square velocity of the nitrogen gas outside the tank
Step-by-step explanation:
Volume of container = 100.0 L
Temperature = 293 K
pressure = 1 atm = 1.01325 bar
number of moles n = ?
using the gas equation PV = nRT
n = PV/RT
R = 0.08206 L-atm-
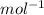

Therefore,
n = (1.01325 x 100)/(0.08206 x 293)
n = 101.325/24.04 = 4.21 moles
The equation for root mean square velocity is
Vrms =
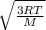
R = 8.314 J/mol-K
where M is the molar mass of oxygen gas = 31.9 g/mol = 0.0319 kg/mol
Vrms =
 = 478.6 m/s
= 478.6 m/s
For Nitrogen in thermal equilibrium with the oxygen, the root mean square velocity of the nitrogen will be proportional to the root mean square velocity of the oxygen by the relationship
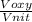 =
=
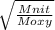
where
Voxy = root mean square velocity of oxygen = 478.6 m/s
Vnit = root mean square velocity of nitrogen = ?
Moxy = Molar mass of oxygen = 31.9 g/mol
Mnit = Molar mass of nitrogen = 14.00 g/mol
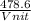 =
=
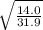
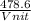 = 0.66
= 0.66
Vnit = 0.66 x 478.6 = 315.876 m/s
the root mean square velocity of the oxygen gas is
478.6/315.876 = 1.5 times the root mean square velocity of the nitrogen gas outside the tank