Answer:
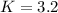
Step-by-step explanation:
Hello,
In this case, for the given equilibrium, we write the law of mass action:
![K=([PCl_3][Cl_2])/([PCl_5])](https://img.qammunity.org/2021/formulas/chemistry/college/8rcik22jasz8hoqc5atedq0qs0zt3lswea.png)
Next, in terms of the change
 due to the reaction extent (ICE procedure):
due to the reaction extent (ICE procedure):
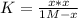
Clearly, the initial concentration phosphorous pentachloride is 1 M (one mole per litre), therefore, since the equilibrium concentration is 0.2 M (same volume) we can compute
 :
:
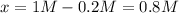
Thus, we compute the equilibrium constant:
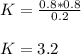
Regards.