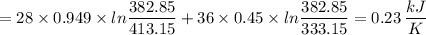Answer:
The equilibrium temperature is 109.7°C
The total entropy change for the process is 0.23 kJ/K
Step-by-step explanation:
The parameters given are;
The initial temperature of aluminium, T₁ₐ = 140°C = 413.15 K
Mass, m₁, of aluminium block = 28 kg
The specific heat of aluminium, c₁, at 400 K = 0.949 kJ/(kg·K)
The initial temperature of iron, T₁ₙ = 60°C = 333.15 K
Mass, m₂, of iron block = 36 kg
The specific heat of iron, c₂, at room temperature = 0.45 kJ/(kg·K)
The heat lost by the aluminium = Heat gained by the iron
The heat lost by the aluminium = m₁ × c₁ × (T₁ₐ - Tₓ)
The heat lost by the aluminium = 28 × 0.949 × (413.15 - Tₓ)
The heat gained by the iron = m₂ × c₂ × (Tₓ - T₁ₙ)
The heat gained by the iron = 36 × 0.45 × (Tₓ - 333.15)
Hence;
28 × 0.949 × (413.15 - Tₓ) = 36 × 0.45 × (Tₓ - 333.15)
26.572 × (413.15 - Tₓ) = 16.2 × (Tₓ - 333.15)
∴ (26.572 + 16.2) × Tₓ = 26.572 × 413.15 + 16.2 × 333.15
42.772·Tₓ = 16375.2518
Tₓ = 382.85 K = 109.7°C
The total entropy change of the process is given by the following relation;