Answer:
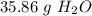
Step-by-step explanation:
In this case, we have to remember that relationship between the avogadro's number and the "mol" concept:
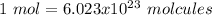
With this in mind, we can do the first conversion:
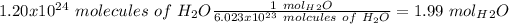
Now, if we calculate the molar mass of
 . The atomic mass of O is 16 g/mol and the atomic mass of H is 1 g/mol, so:
. The atomic mass of O is 16 g/mol and the atomic mass of H is 1 g/mol, so:
(16*1)+(1*2)= 18 g/mol
In other words,
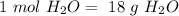 , so:
, so:
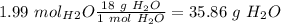
I hope it helps!