Answer :
(a) The name of gas given off in the reaction is hydrogen gas.
(b) When we take the test tube containing zinc and sulfuric acid near a candle or a burner, we hear a pop sound. This shows the presence of hydrogen gas.
(c) The products formed in this reaction are zinc sulfate and hydrogen gas.
(d) When the evolution of hydrogen gas will stop.
(e) The word equation for this reaction is:
Zinc + Sulfuric acid
 Zinc sulfate +Hydrogen gas
Zinc sulfate +Hydrogen gas
Step-by-step explanation:
As we know that when an acid reacts with a metal, then a salt and hydrogen gas are formed.
As per question, when sulfuric acid reacts with zinc metal then zinc sulfate and hydrogen gas are formed.
The word equation for this reaction is:
Zinc + Sulfuric acid
 Zinc sulfate +Hydrogen gas
Zinc sulfate +Hydrogen gas
The balanced chemical reaction is as follows:
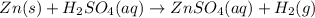
In this reaction, zinc metal displaces the hydrogen form sulfuric acid.
When we take the test tube containing zinc and sulfuric acid near a candle or a burner, we hear a pop sound. This shows the presence of hydrogen gas.