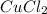Answer: The empirical formula of the compound is
Step-by-step explanation:
Mass of Copper (Cu) = 1.23 g
Mass of Chlorine (Cl) = Mass of copper chloride - mass of copper = (2.61 - 1.23) g = 1.38 g
Step 1 : convert given masses into moles
Moles of Cu =
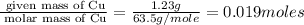
Moles of Cl =
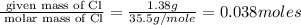
Step 2 : For the mole ratio, divide each value of moles by the smallest number of moles calculated.
For Cu =
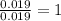
For Cl =
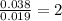
The ratio of Cu : Cl = 1 : 2
Hence the empirical formula is