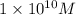Answer: The hydroxide ion concentration in a solution is
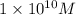
Step-by-step explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration.
![pH=-\log [H^+]](https://img.qammunity.org/2021/formulas/chemistry/high-school/rjo2yhb5oj9ry1fr4db1ujrazm6fh3vhke.png)
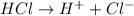
According to stoichiometry,
1 mole of
 gives = 1 mole of
gives = 1 mole of

Thus
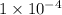 moles of
moles of
 gives =
gives =
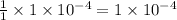 moles of
moles of

![K_w=[H^+][OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/7jdjcuyzjoh0xkeaez8b0dl2066xe1tebt.png)
![10^(-14)=[1* 10^(-4)][OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/2pg9min2gvynru756e2q70rco6gfmhpwbs.png)
![[OH^-]=1* 10^(10)M](https://img.qammunity.org/2021/formulas/chemistry/college/pu3ctykud2td7sdnyfs5mww3e2nasyt44l.png)
Thus the hydroxide ion concentration in a solution is