Answer:
H2SO4 = 0.3885 mol L-1
Step-by-step explanation:
We need (i) a stoichiometric equation:
H2SO4 + 2NaOH = NaSO4 +2H2O
1:2
And (ii) equivalent quantities of sodium hydroxide
Moles of NaOH = 10x
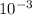 L x 0.777mol
L x 0.777mol
 = 0.00777 mol
= 0.00777 mol
So H2SO4 = (0.5 x 0.00777 mol) / 0.001
= 0.3885 mol L-1