Answer:
1, 1, 3, 1.
Step-by-step explanation:
When balancing chemical equations, the amount of moles of each element on both sides of the equation should be equal.
Looking at the original equation, we can see that there are 3 mols of iron on the reactants side and 1 on the products. We can simply add a coefficient of '3' to 'FeO' to balance the iron.
For Oxygen, we can see 5 on the reactants side. However, since we already added a coefficient of '3' to 'FeO', this already balanced out the oxygen for us. We have 5 mols in the reactants, and 5 in the products.
Carbon is already balanced on both sides.
Therefore, the final formula is
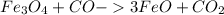 , with coefficients of 1, 1, 3, and 1.
, with coefficients of 1, 1, 3, and 1.