Answer:
Step 1
Work done = -9134.4 J
ΔQ = -9134.4 J
Step 2
ΔQ = -3570.32 J = ΔU
W = 0
Step 3
The pdV work done = 3570.32 J
The Vdp work done = 11053.37 J
Heat transferred, ΔE = 0.
Step-by-step explanation:
For diatomic gases γ = 1.4
Step 1
Where:
v₂ = 3.00·v₁
On isothermal expansion of an ideal gas by Boyle's law, we have;
p₁·v₁ = p₂·v₂ which gives;
p₁·v₁ = p₂×3·v₁
Dividing both sides by v₁, we have;
p₁= 3·p₂
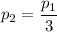
Hence, the pressure is reduced by a factor of 3
Work done =
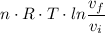
Where:
n = 1 mole
R = 8.3145 J/(mole·K)
T = 1000 K we have
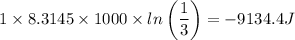
Step 2
The gas undergoes a constant volume decrease in pressure given by Charles law as follows;
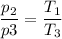
Whereby p₂ > p₃, T₁ will be larger than T₃
W = 0 for constant volume process
ΔQ = m×cv×ΔT = 1 × 3.97 × -900 = -3570.32 J = ΔU
Step 3
For adiabatic compression, we have;
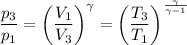
Where:
T₁ = 1000 K
T₃ = 100 K
We have;
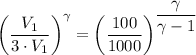
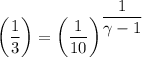
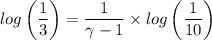
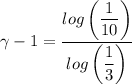
∴ γ-1 = 2.096
γ = 3.096
The pdV work done =
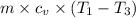
m×R/(γ - 1)×(T₁ - T₃) =
3.97×(1000 - 100) = 3570.32 J
The Vdp work done =
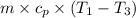
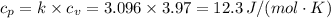
12.3×(1000 - 100) = 11053.37 J
Heat transferred, ΔE = 0.