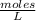Answer:
The molarity of the final solutions if these two solutions are mixed is 27.14
Step-by-step explanation:
Yo know:
- Solution-1: 25M, 400mL
- Solution-2: 30M, 300 mL
Molarity being the number of moles of solute per liter of solution, expressed by:
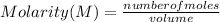
You can determine the number of moles that are mixed from each solution as:
Number of moles= Molarity*Volume
So, being 1 L=1000 mL, for each solution you get:
- Solution-1: being 0.400 L=400 mL ⇒ 25 M* 0.400 L= 10 moles
- Solution-2: being 0.300 L=300 mL ⇒ 30 M* 0.300 L= 9 moles
When mixing both solutions, it is obtained that the volume is the sum of both solutions:
Total volume= volume solution-1 + volume solution-2
and the number of total moles will be the sum of the moles of solution-1 and solution-2:
Total moles= moles of solution-1 + moles of solution-2
So the molarity of the final solution is:
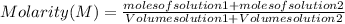
In this case, you have:
- moles of solution-1: 10 moles
- moles of solution-2: 9 moles
- volume solution-1: 0.400 L
- volume solution-2: 0.300 L
Replacing:
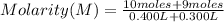
Solving:
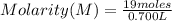
Molarity= 27.14
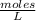
The molarity of the final solutions if these two solutions are mixed is 27.14