Answer:
Compound A: Propylbenzene
Compound B: Cumene
Step-by-step explanation:
On this case, we have two clues:
1) The mass spectrometry info
2) The formation of benzoic acid
If we have the formation of benzoic acid with a strong oxidant (
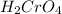 ). It means that we have an alky group bonded to benzene. Therefore we have to add three carbons to a benzene ring in order to obtain the mass of the compound (120).
). It means that we have an alky group bonded to benzene. Therefore we have to add three carbons to a benzene ring in order to obtain the mass of the compound (120).
The next question is how these three additional carbons are bonded to the benzene. For this, we have to check the mass info. For compound A we have a fragmentation moeity on 91 therefore we have a loss of an ethyl group. Therefore compound A have a linear structure for the aditional three carbons.
For compound B we have a fragmentation moeity on 105 this means a loss of 15 units of mass therefore we loss a methyl group. This indicates that we have a non linear structure fo the three additional carbons.
See figures 1 and 2.
I hope it helps!