Answer:
1) 2H2O2=O2+2H2O
2) 0.67
3) 7.86
Step-by-step explanation:
1) Hydrogen Peroxide: H2O2
Water: H2O
Oxygen: O2
H2O2= H2O+O2
Number of Hydrogen's in the reactants side (left side): H2, so two H's.
Number of Oxygen's in the reactants side (left side): O2, so two O's.
Number of Hydrogen's in the product side (right side): H2 so two hydrogens.
Number of Oxygen's in the product side (right side): O+O2, so three oxygens.
The Hydrogen's are already balanced, so we only need to worry about the oxygen's.
If we multiply the H20 part of the product by 2, then we will get 2H2O (remember this also changes the number of H's)
But this is actually good, because now we have two hydrogens and two oxygens on the reactants side, and four hydrogens and oxygens on the product side.
The last step in balancing the equation is to put a two in front of the H2O2, which will double the amount of hydrogen and oxygen in the reactants part.
And the equation is balanced!
2) 1.34 mol H2O2 x
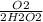
The H2O2's can cancel out, so all we have now is 1.34O2/2 which is equal to 0.67 O2. Thus, 0.67 moles of O2 will be produced.
3) 7.86 mol H2O2 x
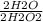
The H2O2's can cancel out, so all we have now is (7.86 x 2)/2, which is just equal to 7.86. Thus, 7.86 moles of H2O2 can form 7.86 moles of water.