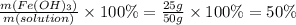Answer:
50%
Step-by-step explanation:
Step 1: Calculate the mass of water
Since we don't know the actual temperature at which the solution is prepared, we will assume the density of water is about 1 g/mL.
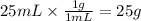
Step 2: Calculate the mass of the solution
The mass of the solution is the sum of the mass of the solute (Iron (III) hydroxide) and the mass of the solvent (water).
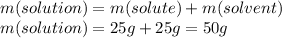
Step 3: Calculate the percent by mass of Iron (III) hydroxide in the solution