Answer:
Step-by-step explanation:
1. Data
Mass of graduated cylinder = 47.229 g
Mass of graduated cylinder + water = 71.821 g
Actual volume of water = 25.0 mL
2. Calculations
(a) Mass of water
Mass = 71.821 g -47.229 g = 24.592 g
(b) Volume of water
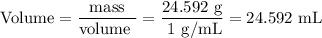
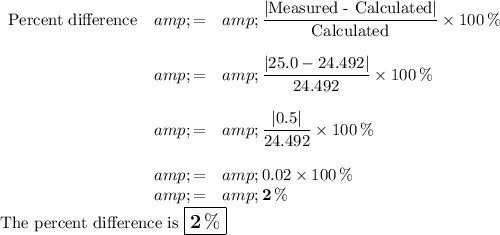
(c) Percent Difference