Chlorine dioxide is used as a disinfectant and bleaching agent. In water, it reacts to form chloric acid (HClO3),
according to the following balanced equation:
6 ClO2 + 3 H2O → 5 HClO3 + HCl
If excess ClO2 is mixed with 18.0 mL of H2O (d =
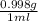
) how many grams of HClO3 are formed?
PLEASE DO NOT JUST GIVE THE ANSWER!!! I really would like to learn HOW to do this so please give explanations for each step as you solve it. Thanks!