Answer:
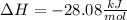
Step-by-step explanation:
Hello,
This types of reactions are likely to be carried out in gaseous phase as it is easier to induce reactions, therefore, for us to compute the change in the enthalpy of this reaction we should write the formation enthalpy of gaseous methanol, hydrogen chloride, methyl chloride and water as -205.1, -92.3, -83.68 and -241.8 kJ/mol respectively. Then, the reaction enthalpy for this reaction is:
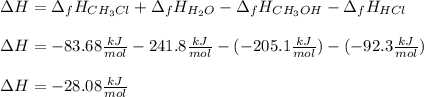
Which accounts for an exothermic chemical reaction.
Regards.