Answer:
About 164 grams
Step-by-step explanation:
The molecular formula of calcium nitrate is
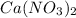 . The molar mass of calcium is about 40, while the molar mass of nitrogen is about 14 and oxygen is about 16. Therefore, the molar mass of it is:
. The molar mass of calcium is about 40, while the molar mass of nitrogen is about 14 and oxygen is about 16. Therefore, the molar mass of it is:
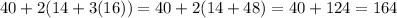
Hope this helps!