Answer:
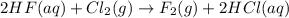 will not occur.
will not occur.
Step-by-step explanation:
A single replacement reaction is one in which a more reactive element displaces a less reactive element from its salt solution. Thus one element should be different from another element.
A general single displacement reaction can be represented as :
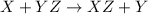
a)
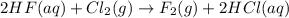
Flourine is more reactive than chlorine and hence this reaction cannot occur.
b)
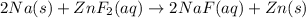
Sodium is more reactive than zinc and hence the reaction will occur.
c)
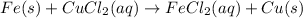
Iron is more reactive than copper and hence the reaction will occur.
d)
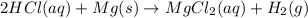
Magnesium is more reactive than hydrogen and hence the reaction will occur.