Answer:
(B) HNO3 + NaOH
Step-by-step explanation:
Hello,
In this case, for the production of sodium nitrate a substance having the sodium as a cation should react with another substance having the nitrate anion. Moreover, neutralization reactions are carried out when bases react with acids, such is the case of nitric acid and sodium hydroxide:
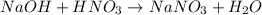
Thus, the answer is (B) HNO3 + NaOH as the other options involve salts rather than acids or bases as starting reactants.
Regards.