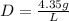Answer: D=4.35g/L
Step-by-step explanation:
The formula for density is
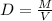 . M is mass in grams and V is volume in liters.
. M is mass in grams and V is volume in liters.
Since we are give pressure and temperature, we can use the ideal gas law to find moles/volume. FInding moles/volume would give us the base for density. All we would have to do is convert moles to grams.
Ideal Gas Law: PV=nRT
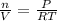
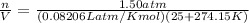
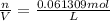
Now that we have moles, we can use molar mass of chlorine gas to find grams.
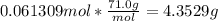
With our grams, we can find our density.
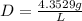
We need correct significant figures so our density is: