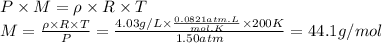Answer:
44.1 g/mol
Step-by-step explanation:
Step 1: Convert the temperature to the Kelvin scale.
We will use the following expression.
K = °C + 273.15
K = -73°C + 273.15
K = 200 K
Step 2: Convert the pressure to atm
We will use the relationship 1 atm = 760 torr.
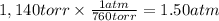
Step 3: Calculate the molar mass of the gas
We will use the following equation derived from the ideal gas equation.