Answer:
the equilibrium constant of the decomposition of hydrogen bromide is 0.084
Step-by-step explanation:
Amount of HBr dissociated
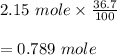
2HBr(g) ⇆ H2(g) + Br2(g)
Initial Changes 2.15 0 0 (mol)
- 0.789 + 0.395 + 0.395 (mol)
At equilibrium 1.361 0.395 0.395 (mole)
Concentration 1.361 / 1 0.395 / 1 0.395 / 1
at equilibrium (mole/L)
![K_c=([H_2][Br_2])/([HBr]^2) \\\\=((0.395)(0.395))/((1.361)^2) \\\\=(0.156025)/(1.852321) \\\\=0.084](https://img.qammunity.org/2021/formulas/chemistry/college/hzpim71pia22sa757efqyxeo9ppwyxppyy.png)
Therefore, the equilibrium constant of the decomposition of hydrogen bromide is 0.084