Answer:
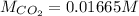
Step-by-step explanation:
Hello,
In this case, the Henry's law allows us to relate the molar concentration and partial pressure of a solute (carbon dioxide) in a solution (solvent is water) by:
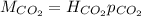
Whereas we introduce the Henry constant, therefore, we can easily compute the molar solubility by:
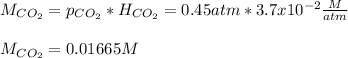
Regards.