Answer:
1.3 M
Step-by-step explanation:
We dissolve 25 g of sodium hydroxide en water until 500 cm³ of solution are reached. What is the molar concentration of the solution?
Step 1: Calculate the moles of sodium hydroxide (solute)
The molar mass of sodium hydroxide is 40.00 g/mol.
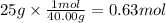
Step 2: Calculate the liters of solution
We will use the following relations:
- 1 cm³ = 1 mL
- 1 L = 1,000 mL

Step 3: Calculate the molarity of the solution
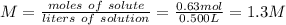
The molarity of the solution is 1.3 M.