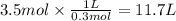Answer:
11.7 L
Step-by-step explanation:
How many liters of 0.3M H₃PO₄ are needed to neutralize 3.5L of 3M NaOH?
Step 1: Write the balanced equation
H₃PO₄ + 3 NaOH = Na₃PO₄ + 3 H₂O
Step 2: Calculate the reacting moles of sodium hydroxide
3.5 L of 3 M NaOH were employed. The reacting moles of NaOH are:
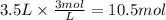
Step 3: Calculate the reacting moles of phosphoric acid
The molar ratio of H₃PO₄ to NaOH is 1:3. The reacting moles of H₃PO₄ are (1/3) × 10.5 mol = 3.5 mol
Step 4: Calculate the required liters of phosphoric acid
3.5 moles of 0.3 M H₃PO₄ were employed. The required volume of H₃PO₄ is: