Answer: Weak acids are poor conductors of electricity.
Step-by-step explanation:
Weak acids are defined as the acids which dissociate weakly or partially when dissolved in water (polar solvent).
For example,
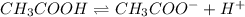
Due to less dissociation there will be less number of ions present in a solution of weak acid. As a result, a weak acid will be poor conductor of electricity.
As electricity is the flow of ions.
Thus, we can conclude that weak acids are poor conductors of electricity.