Answer : The theoretical yield in grams for this reaction under the given conditions is, 8.28 grams.
Explanation : Given,
Mass of
 = 1.46 g
= 1.46 g
Mass of
 = 10.5 g
= 10.5 g
Molar mass of
 = 2 g/mol
= 2 g/mol
Molar mass of
 = 28 g/mol
= 28 g/mol
First we have to calculate the moles of
 and
and
 .
.
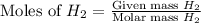
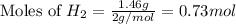
and,
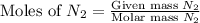
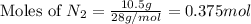
Now we have to calculate the limiting and excess reagent.
The balanced chemical equation is:
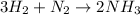
From the balanced reaction we conclude that
As, 3 mole of
 react with 1 mole of
react with 1 mole of

So, 0.73 moles of
 react with
react with
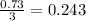 moles of
moles of

From this we conclude that,
 is an excess reagent because the given moles are greater than the required moles and
is an excess reagent because the given moles are greater than the required moles and
 is a limiting reagent and it limits the formation of product.
is a limiting reagent and it limits the formation of product.
Now we have to calculate the moles of

From the reaction, we conclude that
As, 3 mole of
 react to give 2 mole of
react to give 2 mole of

So, 0.73 mole of
 react to give
react to give
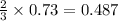 mole of
mole of

Now we have to calculate the mass of

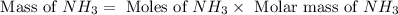
Molar mass of
 = 17 g/mole
= 17 g/mole
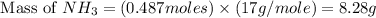
Therefore, the theoretical yield in grams for this reaction under the given conditions is, 8.28 grams.