Answer:
30,298 joules of heat are needed to melt 50 g of ice at 0°C and then warm the liquid to 65°C
Step-by-step explanation:
To analyze the heat that must be supplied to the ice, two phases are analyzed: one in which a part of heat will be required to melt the ice, that is, convert 0 ° C of ice to 0 ° C in a liquid state, and another part of heat that will raise the temperature of the melted ice to 65 ° C. So:
Total heat required = Heat required to melt ice + Heat required to raise the temperature of the ice in liquid state
Being fusion, the process that a substance undergoes to go from a solid state to a liquid, then the heat required to convert 0 ° C of ice to 0 ° C in a liquid state is calculated as:
Heat required to melt ice=mass*heat of fusion of ice= 50 g* 334
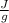 = 16,700 J
= 16,700 J
On the other hand, the amount of heat received or transferred by a body when it undergoes a temperature variation (Δt) without there being a change of physical state (solid, liquid or gaseous) is calculated by the expression:
Q = c * m * ΔT
Where Q is the heat exchanged by a body of mass m, made up of a specific heat substance c and where ΔT is the temperature variation (Tfinal-Tinitial).
Then, the heat required to go from water at 0 ° C to water at 65 ° C is calculated by:
q=specific heat of water*m*ΔT= 4.184
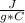 *50 g* (65 °C - 0°C)= 13,598 J
*50 g* (65 °C - 0°C)= 13,598 J
So:
Total heat required = 16,700 J + 13,598 J
Total heat required = 30,298 J
30,298 joules of heat are needed to melt 50 g of ice at 0°C and then warm the liquid to 65°C