Answer: 0.01 M
Step-by-step explanation:
Molarity is moles of solute/liters of solution.
To find moles, we need to convert 0.37 g of KCl into moles.
30.098g/mol K + 35.45 g/mol Cl = 65.548 g/mol KCl
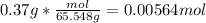
Now that we have moles, we need to convert 500 mL to L.
500 mL = 0.5 L
With our moles and liters, we can find molarity.
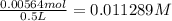
Significant figures is important. Our answer should be 0.01 M.