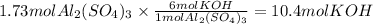Answer:
10.4mol
Step-by-step explanation:
Step 1: Write the balanced equation
Al₂(SO₄)₃ + 6 KOH ⇒ 2 Al(OH)₃ + 3 K₂SO₄
Step 2: Establish the appropriate molar ratio
According to the balanced equation, the molar ratio of Al₂(SO₄)₃ to KOH is 1:6.
Step 3: Calculate the moles of potassium hydroxide needed to completely react with 1.73 moles of aluminum sulfate