Answer: 1.4 moles
Step-by-step explanation:
I can only assume you are looking for the amount of moles in 0.4M. the capital M means Molarity.
Molarity=moles of solute/liters of solution
Since we know the molarity is 0.4, we can plug this into our equation
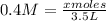
moles= 1.4