Answer: The given reaction is a double displacement reaction.
Step-by-step explanation:
A chemical reaction in which two different compounds chemically react with each other by switching places of both positive and negative ions is known as a double displacement reaction.
For example,
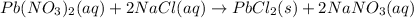
Here, both
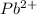 and
and
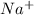 ions switched places. Also, both
ions switched places. Also, both
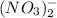 and
and
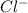 ions switched places.
ions switched places.
Therefore, we can conclude that the given reaction is a double displacement reaction.