Answer:
Step-by-step explanation:
We can use oxidation numbers to decide which substance is reduced.
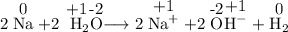
The oxidation number of Na changes from 0 in Na to +1 in Na⁺.
The oxidation number of H changes from +1 in H₂O to 0 in H₂.
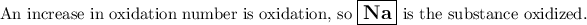
1 and 4 are wrong because H₂ and Na⁺ are products.
2. is wrong because there is no H⁺ to be oxidized or reduced.