Answer : The correct option is (b) 2, 3, and 6
Explanation :
Combustion reactions: It is defined as the reactions in which a hydrocarbon reacts with oxygen gas to produce carbon dioxide and water.
A balanced chemical equation is defined as the equation in which total number of individual atoms on the reactant side is equal to the total number of individual atoms on product side.
The general chemical equation for the combustion is as follows:
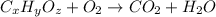
where, 'x', 'y' and 'z' are the subscripts of Carbon, hydrogen and oxygen respectively.
The reactants and products in the same combustion reaction are
 and
and
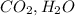 respectively.
respectively.
Hence, the correct option is (b) 2, 3, and 6