Answer:
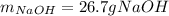
Step-by-step explanation:
Hello,
In this case, given the molality of the solution which is 2.88 m (mol/kg) we can compute the moles of sodium hydroxide that are dissolved in the 232 grams of water (0.232 kg) as follows:
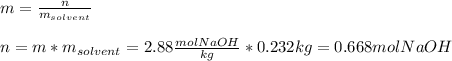
Then, by using the molar mass of sodium hydroxide we compute the grams:
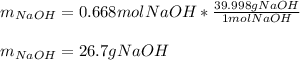
Regards.