Answer:
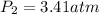
Step-by-step explanation:
Hello,
In this case, we first compute the moles of oxygen in 0.654 g and 1.35 g by using its molar mass (32 g/mol):
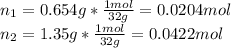
Then, by using the ideal gas equation at the both states, given the same both temperature and volume:
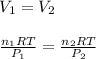
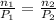
We compute the volume at the second moles:
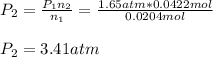
Best regards.