Answer:
The formula of Density for a random unit cell is as follows -
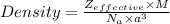
where ,
★
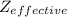 = number of atoms in a unit cell
= number of atoms in a unit cell
★ M = molar mass
★
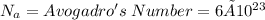
★ a = edge length
now ,
In a tetragonal unit cell ,
the atoms are present over the corners as well as the at the body centre.
Therefore ,
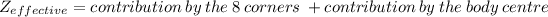
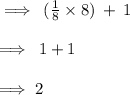
And ,
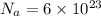
Substituting the values of the following in the formula of Density , we get
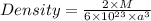
hope helpful :D