Answer:
The de-Broglie wavelength of the nitrogen molecule is 0.0272 nm
Step-by-step explanation:
The mass, m, of the nitrogen molecule, N₂ = 14.0076u = 28.0152 × 1.66 × 10⁻²⁷ kg
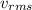 of N₂ = 523 m/s
of N₂ = 523 m/s
The de-Broglie wavelength is given as follows;
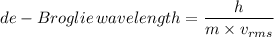
Where:
h = Planck's constant = 6.62607004 × 10⁻³⁴ m²·kg/s
Plugging in the values, we have;
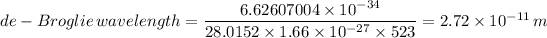
Hence, the de-Broglie wavelength of the nitrogen molecule = 0.0272 nm.