Answer:
 is the correct answer to the given question.
is the correct answer to the given question.
Step-by-step explanation:
Given k=6.40 x 10-3 min-1.
According to the first order reaction .
The concentration of time can be written as
![[\ A\ ]\ = \ [\ A_(0)\ ] * e \ ^\ {-kt}](https://img.qammunity.org/2021/formulas/chemistry/college/euyg1igdy6wu99fta31j14hvx9bd26kn10.png)
Here
![[\ A\ ]_(0)](https://img.qammunity.org/2021/formulas/chemistry/college/liu81pq3q6pcra4iso2y3yfka3ckx7egnj.png) = Initial concentration.
= Initial concentration.
So
![[\ A\ ]_(0)= 0.0314 M](https://img.qammunity.org/2021/formulas/chemistry/college/umrkkfjll350jkaexmbro7id2yftc0n5hr.png)
Putting this value into the above equation.
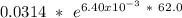
=0.211 M
This can be written as
