Answer:
the volume of the gas at conditions of STP = 151.04998 ml
Step-by-step explanation:
Data given:
V1 = 180 ml
T1 = 35°C or 273.15 + 35 = 308.15 K
P1 = 95.9 KPa
V2 =?
We know that at STP
P2 = 1 atm or 101.3 KPa
T2 = 273.15 K
At STP the pressure is 1 atm and the temperature is 273.15 K
applying Gas Law:
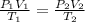
putting the values in the equation of Gas Law:
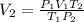
V_2 =
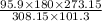
V2 = 151.04998
therefore, V2 = 151.04998 ml